Abstract
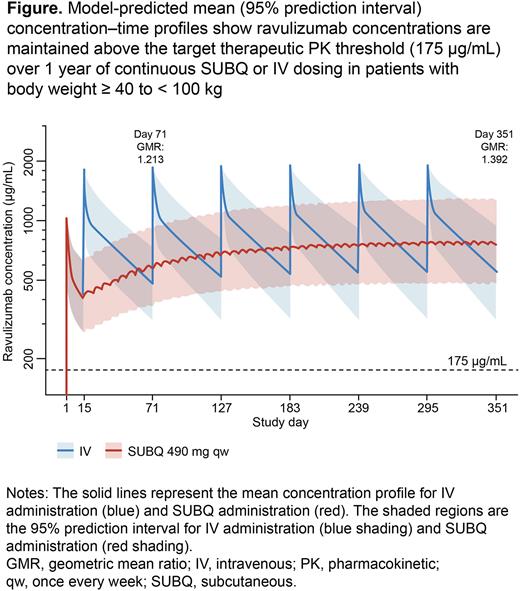
Background: The efficacy of ravulizumab (intravenous [IV] formulation administered every 8 weeks) for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) has been demonstrated in randomized trials (NCT02946463, NCT03056040, NCT03406507, NCT02949128). Studies of IV ravulizumab have established the target therapeutic pharmacokinetic (PK) threshold of 175 μg/mL for complete and sustained terminal complement inhibition (free C5 < 0.5 µg/mL) in patients with PNH and aHUS; maintenance of serum ravulizumab trough concentration (Ctrough) above this threshold ensures clinical efficacy. In study 303 (NCT03748823), the on-body delivery system for subcutaneous (SUBQ) ravulizumab administration (490 mg self-administered weekly) showed PK non-inferiority to IV ravulizumab after 71 days of treatment in adult patients with PNH weighing ≥ 40 to < 100 kg who were clinically stable on prior IV eculizumab treatment (Yenerel et al. HemaSphere 2021;5:e566). In this study, a population PK (popPK) model was developed to characterize ravulizumab PK following IV or SUBQ ravulizumab treatment in patients with PNH, which was then used to generate model-based dosing simulations to support SUBQ and IV ravulizumab posology.
Aims: To develop a popPK model and perform simulations to evaluate model-predicted 1) ravulizumab concentrations following IV and SUBQ administration over 1 year of treatment, 2) ravulizumab exposures following SUBQ administration in patients weighing ≥ 100 kg and 3) impact of switching administration route (from IV to SUBQ ravulizumab and vice versa).
Methods: A popPK analysis of ravulizumab was performed using data from patients enrolled in three clinical trials (NCT05288829, NCT05396742, NCT03748823). Concentration-time profiles of ravulizumab following IV and SUBQ administration were simultaneously modeled with a 2-compartment model with linear clearance. Ravulizumab absorption following SUBQ administration was modeled using a first-order rate constant of absorption (Ka), bioavailability and lag time. Model-based analyses for repeated measures PK endpoints were conducted using nonlinear mixed effects modeling. The final popPK model accounted for covariates such as body weight, body mass index, disease status and hemoglobin, and was used to simulate steady state concentration profiles following dosing over 1 year, exposure in virtual patients weighing ≥ 100 kg and the impact of a switch in administration route after differing durations of dosing.
Results: In total, 2689 blood samples (SUBQ: 1728; IV: 961) were obtained from 178 participants (129 with PNH and 49 without; 115 treated with SUBQ and 63 IV ravulizumab; 46.6% female; aged 18-79 years; body weight 43.5-98.4 kg). The mean (standard deviation) terminal elimination half-life of ravulizumab after SUBQ dosing was 52.4 (9.72) days. The bioavailable fraction of ravulizumab after SUBQ administration was 79.4%. The model-predicted geometric mean ratio (GMR) of SUBQ compared with IV Ctrough on Day 71 and 351 (steady state) were 1.213 and 1.392, respectively (Figure) and comparable to the Day 71 GMR of 1.257 in study 303, indicating the developed popPK model accurately characterized ravulizumab exposure for the ≥ 40 to < 100 kg body weight range in the trial. Simulations predicted that SUBQ ravulizumab Ctrough both remained above the target PK threshold and was non-inferior to ravulizumab IV for body weights ≥ 100 kg. Simulations describing a switch in ravulizumab formulation (IV to SUBQ or SUBQ to IV) show all concentrations remained within our target exposure range regardless of dosing order, after a single dose or after 6 months of dosing.
Conclusions: A popPK model was developed and used to support an expanded indication and unrestricted dosing interchangeability between SUBQ and IV ravulizumab. Simulations using the popPK model predicted that switching SUBQ or IV ravulizumab administration route still maintained ravulizumab concentration within the target exposure range supporting treatment flexibility for patients receiving ravulizumab, and that the studied dose regimen maintained ravulizumab concentrations above the PK threshold for patients with body weight ≥ 100 kg. This study illustrates the utility of popPK modeling and simulation to supplement clinical trial results in expanding the target population and dosing options for patients with rare diseases.
Disclosures
Ortiz:Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in private company. Monteleone:Alexion, AstraZeneca Rare Disease: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.